Why do we blame big pharma and not the DEA or FDA?
The Opioid Litigation as an Impeachment of Federal Regulators
A desire to blame big pharma opioid manufacturers, distributors, and retailers for our opioid crisis drives much of the dragnet of its associated civil litigation — both in the Judge Polster-led multi-district litigation (“MDL”) in federal court, where plaintiffs are city, county, and tribal governments; and in state attorneys general (AG)-led suits in state courts, where plaintiffs are states.
“With the privilege of lawfully manufacturing and distributing Schedule II narcotics—and thus enjoying the profits therefrom—comes the obligation to monitor, report, and prevent downstream diversion of those drugs.”
According to those government plaintiffs, big pharma aggressively oversupplied a despairing America with 100 billion pain pills chemically engineered to mimic the highs and lows of recreational heroin use, generated black market demand for illicit opioids, and ultimately “caused” 400,000 Americans to perish from opioid overdoses. To them, big pharma opioid manufacturers, distributors, and retailers ought to be held accountable for vitiating their contract with society at large to maintain the careful, détente-like balance between the pursuit of healthcare profits and upholding the public’s health.
“This is an industry that’s out of control. If they don’t follow the law in drug supply, and diversion occurs, people die. That’s just it, people die. … And what they’re saying is, ‘The heck with your compliance. We’ll just get the law changed.”
Government officials hound on big pharma’s “moral, if not legal obligation” to abate the costs of our crisis. Should it mean anything, though, that big pharma opioid companies owe their fiduciary duties not to us, but to their shareholders?
We snack on our fantasies of disengorging billions from big pharma opioid manufacturers, distributors, and retailers, partly because our karmic sense of balance entails that they deserve it. But we also do it to stave off the devastating fictionality of recompense and legal wholeness when people are losing loved ones rather than property. What would make a mother — grieving a son deceased to his opioid use disorder, a condition enabled by the capitalistic gluttony of an industry that prefers profit over the public’s health — whole?
And what would recompense a nation left to sue corporations to abate a crisis exacerbated by the failures of their federal regulators? Observing capitalism wreak havoc on the health and wellbeing of everyday Americans — and the FDA and DEA bow to the wishes of the pharmaceutical lobby — feels disturbingly like watching ethical coastlines disappear. And to fully acknowledge this dystopia forces us to accept that the extraction of hefty settlements via litigation may be one of the few tools we have left to arraign wayward industries entirely incentivized to hijack both their regulators and legislators as their common business practice. In a society inured over decades to “accept[ its] multiparty health system with a significant profit motive,” massive civil settlements, according to Yale law professor Abbe Gluck, may be “the only way to get relief in anyone’s lifetime. [They’re] just more practical.”
Indeed: the supersized scope of the opioid litigation is rendered even more American by our faith in a reckoning through money. But suing a bunch won’t make America feel great again either, as we’re told that best-case global settlement offers wouldn’t even come close to matching the costs governments have borne already to stem the tide of overdoses in their communities. Any civil settlement would allow big pharma opioid companies to exit the stage of legal culpability without having to admit the entirety of their villainy out loud. And even if we were to hold all parties accountable — say, specific members of the Sackler family, the DEA attorneys who opted to slap companies on the hand rather than shackle them, the members of Congress who accepted drug lobby cash in exchange for support of a bill that made it harder for regulators to stop suspicious orders, the FDA officials who took one-to-one meetings with Purdue’s executives prior to approving Oxy’s 2001 label change — the remaining healthcare infrastructure, with its “gaps in access,” “high cost[s],” “poor quality” and “cultural competence in provision of physical, mental, and behavioral health services” would remain. Which is to say nothing of “wages, marriage rates, job quality, social cohesion, cultural capital, and, perhaps, racial privilege” that “ostensibly dr[ove] an ever-larger number of non-college-educated whites into suicidal” or addictive behaviors.
We take to strong stances about retribution in this crisis because the instinct to blame somebody for this crisis is strong. It’s easier to hate big pharma for its capitalistic offenses than to confront our disappointment at the federal government’s devastating failures of defense.
But what we risk by focusing our blame on big pharma and writing off the desire for governmental accountability as folly is a candid discussion of the contextual failures that allowed this crisis to occur.
Below is a year-by-year breakdown of the specific decisions illustrating big pharma’s slow, creeping capture of its regulators. The horizontal axis chronicles the years of our crisis, while the vertical axis charts annual overdose deaths involving opioids:
CENTURIES OF WISDOM, TOPPLED
While the 21st century American opioid crisis might be our species’ most egregious, it certainly isn’t its first. Our love for opioids is as old as society itself. “Prehistoric evidence suggests that opium use may date as far back as fifty thousand years ago, based on residue of opioids in Neanderthal settlements.” Humans have been cultivating opium poppies for over five thousand years. And Western civilizations in modernity have historically experienced massive upticks in opioid-related fatalities when experiencing a type of macro-socioeconomic trauma that destabilizes its political majority — a theory I better explore in “The Cure for America’s Opioid Crisis? End the War on Drugs” (with Steve P. Calandrillo, Harvard Journal of Law & Public Policy 2019).
A type of common-sense opiophobia — a “plague” to those who saw it as a barrier to profit — is what initially prevented pharmaceutical companies from reintroducing “blockbuster opioids” into the modern healthcare marketplace. Heroin and morphine were criminalized or otherwise restricted in the United States since the 1920s, but before the creation of our modern drug statutes, centuries of overdose deaths, trial, and error are what used to ensure that “any idea that there could be such a thing as a non-addictive opioid” received “very fierce counterattack.”
The administrative state operationalizes this opiophobic milieu through its federal regulatory regime, which is designed in part to protect the public against the excesses of capitalism as applied to medicine. The Food & Drug Administration (FDA)’s stated mission is to protect public health, and the Drug Enforcement Administration (DEA)’s to enforce the Controlled Substances Act. Together, they are tasked to maintain a “closed system” of prescription opioids that are supposed to be ensured — pre-market, even — to be safe and effective for their labeled uses. And this regulatory framework is ideally supposed to hum in silence, bearing the type of janitorial invisibility reserved for systems that simply work.
But this system didn’t work. Big pharma’s successes marketing opioids to the general public are astonishing not only for distracting us from our centuries of opioid-related instincts, but for rendering regulatory oversight null. How did legal opioid dealers disrupt physicians’ opioid prescribing habits, and corrupt a regulatory system so thoroughly that “the same number of legitimate patients, overprescribed patients, and illegitimate patients accessing the drug from the same prescribers” were ensured for years to come, and regardless of regulatory climate?
FEDERAL REGULATORS, CAPTURED
“The Sacklers and Purdue tapped into this need for pain relief and made enormous amounts of money in the process. But they aren’t the only ones. Big pharma is much more than one company. It’s a vast network built on strategic pharmaceutical marketing, lack of government oversight and a whole lot of money.”
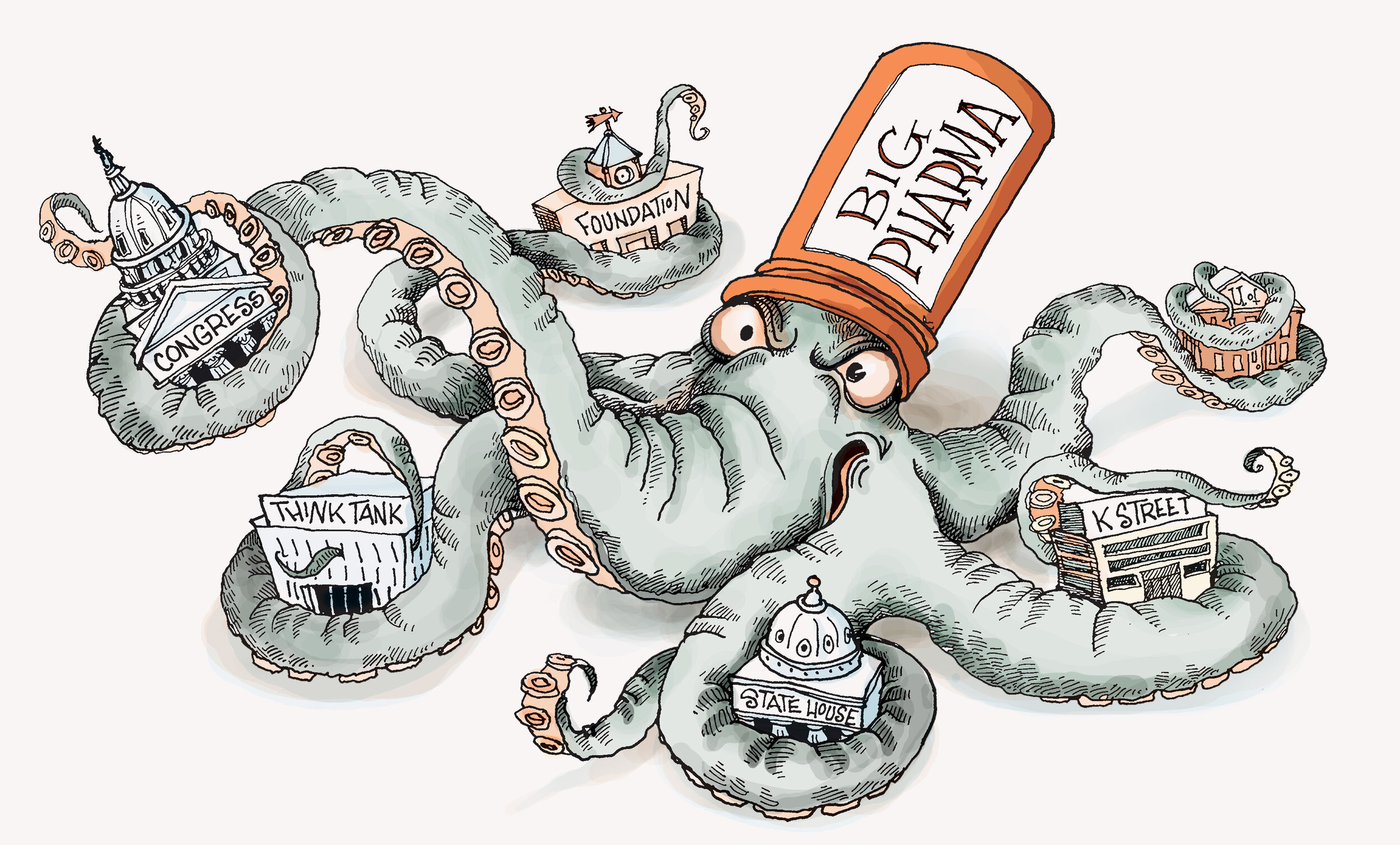
The “backroom dealing, placement of industry-friendly individuals in key regulatory positions, and the breakdown of the regulatory process” required to do so can be best described as regulatory capture. Regulatory capture occurs when the interests of industry and regulators diverge, and dominant industry players begin grooming their regulators over time to turn away from (or respond permissively to) their profiteering and philandering on the things they shouldn’t. Vis-à-vis the opioid crisis, the thing not to be philandered on was the public’s health.
If you take it as a given that the Sackler family — private owners of Purdue Pharma — started the opioid crisis, regulatory capture is essentially how they operationalized their oligarchical, villainous pursuit of profits to topple regulators and seduce key industry players into “fail[ing] to identify suspicious orders []as their business model[s].”
Regardless of who you blame, the term fairly describes the process with which opioid pharmaceutical companies carefully choreographed efforts to shift physicians’ prescribing habits through a multi-year, “coordinated campaign … to convince doctors to prescribe more painkillers, patients to consume more drugs, and regulators to slacken enforcement.”
Slackening an enforcement regime becomes easier when federalism divvies up regulatory authority between entities at different levels of government. Our American system of government “intentionally divide[s]” the “power and authority to govern … between the federal and state governments, with specific responsibilities delegated to each governmental unit.”
State statutes govern the practice of medicine, for instance, which includes the licensure of medical professionals and “the prescribing and dispensing of prescription drugs by licensed health care professionals.” State prescription drug monitoring programs are criticized for maintaining inaccurate data in silos, “preventing linkage across different sectors of state and local governments,” and obscuring the “holistic picture[s] of the relationship[s] between one social issue (e.g., illegal drug prescription and use) and another (e.g., an increased demand for foster care…).”
“Two agencies in particular, the Food and Drug Administration (FDA) and the Drug Enforcement Administration (DEA), are highly accountable for regulating the legal prescription drug industry. The FDA is responsible for regulating prescription painkillers prior to their entrance into the market, whereas the DEA regulates the manufacture, distribution, and possession of prescription medications once they are released to the public.”
But within our federal system of government, state regulators are designed to stand down to federal agencies like the FDA and DEA, who “regulate on behalf of all states” all of the “other responsibilities related to the opioid epidemic.” The FDA and DEA are legislatively charged to ensure the safety of prescription drugs pre-market, and for maintaining a closed system of controlled substances, respectively. And states are genuinely unable to obstruct these agencies’ “Congressionally-given charge[s]” by, say, “interpos[ing] [their] own conclusion[s] about [a drug’s] safety and effectiveness” against federal opinion — a travesty given
Below are those decisions by big pharma set against their broader, federal regulatory context. Click to expand:
Key FDA Failures
Former FDA commissioner David Kessler states that when OxyContin was initially approved for short-term use, the agency’s approval processes lacked “[t]he rigorous kind of scientific evidence that the agency should be relying on,” and that there still, as of today, exist “no studies on the safety or efficacy of opioid for long-term use.” Whether the FDA’s approval processes account for the likelihood of addiction at all is “unclear,” as evidenced by its initial failure to “identify … significant addictive risks and associated sequelae” prior to OxyContin’s release into the market.
What is very clear, however, is that “[a]t the time that OxyContin was first marketed, there were no industry or federal guidelines for the promotion of prescription drugs.” This is in spite of the fact that opioid drug marketing is known to be so “significantly associated” with opioid overdose mortality that it runs directly “counter [to] … national efforts to reduce the number of opioids prescribed.” Drug manufacturers consider a drug’s label to be its “single most important document,” as its language determines “whether somebody can make $10 million or a billion dollars” from it. So, when the FDA approved OxyContin’s revised, 2001 label for “around-the-clock” pain relief — with zero new science but with heavy pressure from pharma — it was, as Dr. Kessler put it, a veritable “marketing tsunami” for big pharma, and certain death to swaths of a nation craving analgesic relief.
With this “blank check” to “push opioids to tens of millions of new pain patients nationwide,” opioid makers “cashed in for billions and billions of dollars.” And this regulatory gap-exploiting genius was enabled in part by the corporate mercenaries who jumped between government and industry positions with ease. “[N]ews stories have documented how FDA employees who worked on opioid regulation accepted high-paying jobs with Purdue,” and “[t]he two medical officers who originally approved Oxycontin, Curtis Wright and Douglas Kramer,” actually “went to work for the opioid maker … not long after leaving the FDA.” But the practice extends beyond the reach of on particular company, as a “large number of key FDA regulators who went through the revolving door to jobs with drug manufacturers” — a practice that, while “suspicious,” remains legal.
Also legal under FDA regulations at the time were the more dubious marketing attempts made by the more philandering big pharma companies. Former employees of Insys, makers of “powerful fentanyl spray” Subsys, testified to the “habit of hiring attractive women as representatives to boost [drug] sales.” One of the women hired under this model “once gave a lap dance at a Chicago nightclub to a doctor Insys was pushing to write more prescriptions,” while other reps were shown motivational “rap video[s]” of “employees danc[ing] and rapp[ing] around a person dressed as a giant bottle of the fentanyl spray.”
The lethal dose of fentanyl remains, as ever, the size of four grains of salt.
Key DEA Failures
“The drug industry, the manufacturers, wholesalers, distributors and chain drugstores, have an influence over Congress that has never been seen before.”
When the opioid crisis began, the DEA was required to consider only “certain factors” when setting opioid production limits, like “past sales and estimated demand.” The 2018 Opioid Quota Reform Act eventually required the agency to consider “the impact of such opioid production on diversion, abuse rates, or overdose deaths,” but not before the DEA permitted 100 billion opioid pain pills to saturate the country from 2006 to 2014, a period in which “more than 130,000 Americans died from prescription opioids.”
The DEA did go after specific big pharma corporations shipping suspicious orders, only to have them flout and re-flout warnings. McKesson repeatedly ignored enforcement efforts between their $13.2 million settlement 2008 to their $150 million settlement with the Justice Department in 2017 — the latter of which was considered a win by the DEA attorneys who negotiated it, but a failure by the DEA investigators who pushed for a $1 billion fine and criminal charges after building the case for years. A $150 million fine is merely “$50 million more than the compensation last year for McKesson board chairman and chief executive John H. Hammergren, the nation’s third-highest-paid chief executive.” Which makes the thrice-flouted warnings of another pharma company, Cardinal Health — whose $34 million in 2008 penalty was quickly followed up by another DEA settlement in 2012, and a $44 million penalty in 2016 — entirely unsurprising.
Unsurprising also are the revolving door-related problems that plague the DEA, which work to strengthen pharma’s regulatory and legislative capture. (“If you want to understand how we were doing our investigations,” says Joseph T. Rannazzisi, the DEA’s former head of diversion, “the best way to do it is to take our people who are doing the investigations and put them in place in your company.”) As the crisis progressed, and as “pharma spent hundreds of millions lobbying Congress,” over fifty DEA and DOJ officials were poached by big pharma companies and the law firms that defend them. The “crowning achievement” of these efforts occurred “in April 2016, at the height of the deadliest drug epidemic in U.S. history,” when “Congress effectively stripped the Drug Enforcement Administration of its most potent weapon against large drug companies suspected of spilling prescription narcotics onto the nation’s streets.”
According to Rannazzisi, for big pharma opioid companies “to get Congress to pass a bill to protect their interests in the height of an opioid epidemic” illustrates “just … how much influence they have.”
DESTINATION “ROUGH JUSTICE”
“Whether settled out of court or litigated to an ultimate finding of liability, however, civil justice in a matter such as this can be only rough justice. Responsibility for the opioid disaster extends well beyond the corporate makers and distributors of the pills, to include prescribers, pharmacists and regulators. In many ways, the opioid crisis illustrates what economists call regulatory capture: Gatekeepers in both the public and private sectors bought into the idea, promoted by industry and by many doctors, that pain was vastly undertreated and that new, extended-release opioid formulations would not be addictive.”
Each of the decisions above might be explained away as mere symptoms of an industry destined for problems from the beginning. Healthcare is “‘big business’” in the States, “with many professionals, organizations, health systems, insurers, and product and service suppliers” accustomed to “significant profits.” And the federal government agrees: the Federal Trade Commission (FTC) and the Department of Justice (DOJ) go as far to say that competition in the industry is “ruthless” and creates “cognitive dissonance” for those “who prefer to focus on the necessity for trust and the importance of compassion in the delivery of health care services” — a statement that prompts the rest of us to question what the “care” in “healthcare” could possibly mean otherwise.
The opioid crisis is a cataclysmically devastating crash of myriad factors, some of which are certainly the creation of private industry alone. But as cash-strapped jurisdictions sue big pharma opioid companies for exacerbating our demand for a drug they were happy to oversupply, and as the litigatory posturing of plaintiffs in our opioid litigation verges on policy theater, where opposing settlement expresses that one is, according to one plaintiffs’ attorney and former DEA enforcer, “hard on white collar crime,” let us not forget those united federal agency failures — the FDA’s and DEA’s in particular — that allowed our epidemic to occur.